Published date: Aug 26, 2018 Tags: plant derived mannose d-mannose 3458-28-4 Non-GMO NAD plant source plant origin China Manufacturer China GMP factory
What is D-Mannose
D-mannose, is a sugar monomer of the aldohexose series of carbohydrates, existing in many kinds of fruits and plants, it is important in human metabolism especially in the glycosylation of certain proteins.
As a natural bioactive monosaccharide, D‐mannose is a popular nutritional and health‐beneficial food supplement, especially used as a dietary supplement influencing glyconutrient contribution on human health. The particular components in D-mannose and its method of absorption in the body make it effective in treating or preventing infections in the bladder and urinary tract.
Our product is from natural extraction instead of chemical synthesis, microbial fermentation or biological transformation. Plant derived, no GMO source D-mannose has low risk , specifically for biopharmaceutical use, It can serve as a key component to ensure consistency of therapeutic glycosylation and potency.
Properties
| CAS No: | 3458-28-4 |
| EINECS: | 222-392-4 |
| Alias Name: | D-(+)-Mannose; Carubinose; Seminose |
| Chemical Structure: | 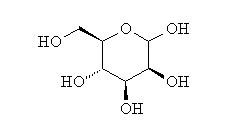 |
| Molecular Formula: | C6H12O6 |
| Molecular Weight: | 180.16 |
| Assay: | NLT 99.0% |
| Solubility: | Easily soluble in water, slight soluble in alcohol |
| Specific Rotation: | +13.7º to +14.7º |
| Application: |
Food additive
Dietary supplement ingredient
Cosmetic ingredient
Agriculture
Start material for synthesis
Reagent
|
Application
Food additive
D-Mannose could be an alternative sweeteners to sucrose for its sweet‐taste and low calories, D‐Mannose has been commercially used in the food industry, it has been added to commercially ice‐cream, processed fruits and salad dressings for its properties , such as improving food texture (solubility, anti‐melting capability).
Dietary supplement
Some clinic results indicated that D-Mannose could be a useful agent for the prevention of recurrent urinary tract infections, on market, it already has many dietary supplements for direct consumption using D-Mannose as single ingredient or with Cranberry extract together. Antibiotics tend to kill both harmful and helpful bacteria. A natural method may help alleviate the discomfort of mild urinary tract and bladder infections.
Cosmetic
d‐mannose is an auxiliary moisturizing agent that is widely used in skin‐care products.
Pharmaceutical industry
D-mannose, is a start material for synthesis of Biotin, Castanospermine, Oseltamivir phosphate. and on november 2018, "Mannose impairs tumour growth and enhances chemotherapy", an new reseach published on Nature indicates that mannose reduces the growth of tumour cells by impairing the metabolism of glucose, and enhances cell death when used in combination with conventional chemotherapy.
Yixin's advantages
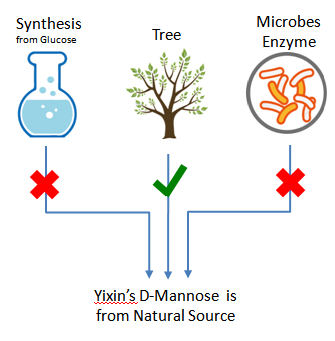
Plant-derived, 100% Non-GMO Source
Yixin’s plant derived D-mannose is completely from nature tree, non GMO source. It does NOT have any GMO risk, neither Yixin nor any of Yixin suppliers of raw materials has used Genetically Modified Organisms in this product.
 |
|
GMP System
Yixin plant derived D-mannose is produced under GMP system to ensure the traceability and consistent supply. As a major dietary supplement ingredient, Yixin D-mannose facility was assessed by NSF to meet GMP requirement for dietary supplement ingredient.
-In 2015-2018 Yixin Carbohydrates facility was assessed by NSF international and found to be in compliance with GMP Requirements in NSF/ANSI Standard173,Section B Dietary Supplements.
-Yixin Carbohydrates Facility was inspected in 2015 by US.FDA, it’s concluded to meet the related requirement of Dietary Supplement Ingredient.
-In 2015-2018 Yixin Carbohydrates facility were certificated by 3rd Kosher certification organization.
-Since 2002, Yixin has obtained the Certificates of GMP from CFDA for 9 dosage forms successively including capsules, tablets, syrup, preparation for external use and etc. In 2006 and 2011 Yixin facility of API was inspected and classified as acceptable by US FDA.
 EN
EN CN
CN DE
DE FR
FR JA
JA RU
RU